Many know that the car battery is important, but few understand how it is able to provide the electric current to the car.
When you look at the car battery, it appears as a simple box that cannot do much, yet powers the car in one of the most powerful ways ever seen.
You see your car stereo work; your installed speakers work just as well or even you might have the brightest lights.
If you like to learn more about this topic, I will explain how a car battery works below.
TABLE OF CONTENTS
Car Batteries Explained
A battery is a device that transforms chemical energy to electric energy and that is the basic way a car battery works.
The chemical energy results from the lead-acid reaction that begins the whole process.
As such the car battery is categorised as a “SLI” which means “starting, lighting and ignition.”
Which primarily explains the role of car batteries which is starting of the car engine, lighting car lights and igniting the car engine.
A car battery is quite different from a normal battery used in remote controls and even in wall clocks.
The batteries for the latter, for the wall clock and the remote controls can be regarded as cells as they are the smallest unit which cannot be disassembled as electrochemistry units.
However, the car battery is a combination of six similar cells that are arranged in a serial manner and the voltages of each is added and contributes to a greater total voltage.
For the car battery, the voltage reading is 12V. The explanation here is for the basic way as to which a car battery is able to function.
How Do Car Batteries Work?
This is a question that can be answered with a little bit of chemistry and physics on electrochemistry.
Electrochemistry is a theory in which electric current is produced from a chemical reaction or a vice versa case.
For the car battery, there is a chemical reaction that results to electric current that powers the car.
In order to understand the operation of the car battery, you have to know what the car battery is made up of.
Car batteries are composed of cells which are the smallest units of electrochemistry.
These cells are six in number, have two plates known as grids and function independently but similarly to one another.
The plates are made of lead and lead dioxide and are designed to produce 2V each, which results to 12V in total hence the reason why car batteries are regarded to as 12V batteries.
The grids are submerged in an electrolyte which is sulphuric acid that acts as the catalyst in this reaction.
The presence of the acid kick starts a reaction in which leads to a chemical reaction in the car battery.
This is explained below:
The sulphuric acid reacts with the lead dioxide plate and this will lead to the production of a sulphate and ions.
The ions move on and react with the other plate, the lead plate and this leads to production of hydrogen and lead sulphate.
Through this chemical reaction electron are produced that race around the plates, or rather the grids and thus generate electricity.
This is the electricity that runs through the battery cables to the car and powers the engine and the electronics.
This reaction, as you should know, is completely reversible.
This is the reason as to why you are able to jumpstart the car battery and charge it as it is being used up to the time it will demise.
Lead and lead dioxide can be formed on the platys once again after current has been provided to the car battery at the appropriate voltage.
This then enables you use the car battery for another discharge cycle.
The Process of Recharging the Car Battery
As described above the process as to which the car battery uses to produce electric current can be reversed entirely.
When the car battery is being used the lead and lead dioxide grids tend to be used up and if this continues for long, the car battery will not be able to produce as much voltage as it could initially.
The reverse process is what is used as a principle for the recharging of the car battery.
Here the plates rea recreated to an extent that when the car battery needs to be used, it will be able to perform well because of the recreation of the structure within it.
After charging everything goes back to normal, there will be one plate or grid of lead sulphate and the other of pure lead.
The electrolyte will contain a sulphuric acid that is more dilute than the way it was initially on being drained.
However, this is not to mean that a car battery will be able to last forever, rather the losses that are associated with these reactions are time bomb that mark the lifetime of the car battery.
The juice box is only able to withstand a certain number of recharging cycles before it is fully dead.
Therefore, it is advisable that you regularly recharge your car battery even before it gets fully drained to increase the chances of it being reliable for a very long time.
What Percentage Does A Car Battery Need To Start?
A car battery should be at approximately 75% for it to even start the vehicle engine. A car battery requires a certain minimum percentage of current to start.
This is usually dependent on a number of factors which include the engine type of the car, engine size and the temperature conditions.
For instance, on a cold day, you will need your car battery to be well charged for it to even start the car.
The battery might have to produce thrice as much current to power the car as compared to a normal day.
This is because of a greater cranking load that is caused by the engine oil being thicker on a cold day and a cold engine requires more power to start.
What Are the Functions Of A Car Battery?
The car battery is the “juice box” of the vehicle and without it or it being in peer condition the vehicle would not be able to perform as needed.
The main role of the car battery in the vehicle as many know is that it provides power for the starting and operation of the car engine.
The car voltage would be at a certain percentage for the engine to start when you even switch on the ignition, otherwise it will not be able to work well.
Many car owners get to realize the importance of a car battery when they get stranded by the roadside with a dead car battery.
The car battery is also in charge of powering up the electronics in the car like the stereo, AC system and even the headlights.
Some car owners fit computers and even monitors in their car which rely on the car battery to be in good condition for them to work well.
Having these on for long periods discharges the car battery and make it unable to perform its main role of powering the engine, and this is bad when the car is idle and the alternator is not working to recharge the car battery as it is being discharged.
What Starts The Battery In A Car?
Many would think that the engine is what starts the car, but this cannot be further from the truth.
The car battery is started by the action of switching the ignition key after putting it in place in the car.
Some modern cars are designed that you just push a button and the car will have been.
Either of these actions sends a signal to the car battery that is in the trunk of the car. This provokes that car battery to act.
Are Car Batteries Rechargeable?
Yes, car batteries are the most common recyclable sources of energy.
In fact, a car battery can be recharged even as power is being drawn from it while you are driving.
This is accomplished by an alternator that is fitted and connected to the car battery.
An alternator in good condition constantly works to provide charge to the car battery as it is working, simply because there is a significant drain from the car battery as the car is running.
This arises from the powering of the car engine as well as other electronics.
If the car battery is unable to recharge when fitted in the car, then you might be dealing with a situation of a damaged alternator.
Another possibility might be because of parasitic drain in the car system. A technician should be consulted to remedy the situation early enough.
A car battery can also be recharged with a good type of car battery charger.
Such types of chargers are designed in a manner that you could set the rate at which the car battery is being recharged and you could even know what time the charging can be completed.
A car battery that might not be able to hold charge after being effectively charged with a good charger might just be damaged.
What Is A Car Battery Made Of?
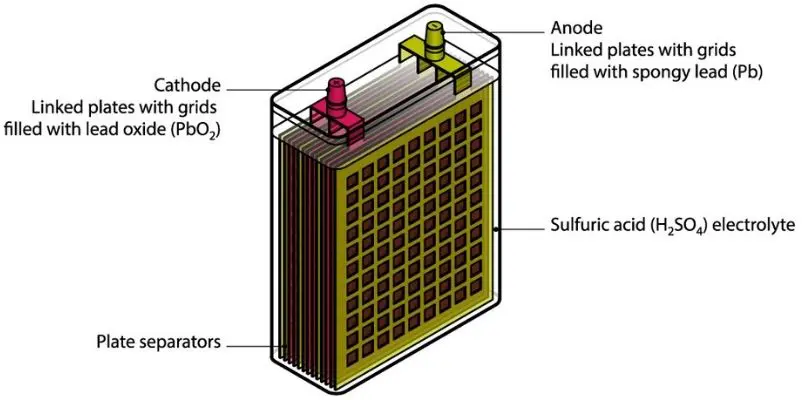
A car battery is made of cells, that are the tiniest electrochemical units, meaning you can’t divide or disassemble them to any lower unit than that.
The car battery is designed with six cells, each of the cell having two plates; a lead and a lead dioxide plate.
The plates are then submerged in sulphuric acid, the electrolyte, which acts as the catalyst reacting with the plates and producing hydrogen and electrons.
The electrons are what move around in the cell and result into the electric energy we see powering the vehicles we drive.
On the outside the car batteries are manufactured with a tough casing that prevents whatever is inside from leaking or accidentally coming out.
This is important as the components in the interior can be quite harmful and are pollutants to the immediate environment.
It will take a heavy hit for a crack or even a bulge to form on the car battery, which will make you to have to replace the car battery altogether.
On the outside you should also be able to spot terminals that are usually on the upper side of the car battery.
How Many Cells Does A Car Battery Have?
When you explore and get to open a car battery, you will be able to see six cells, that is in the case of a typical automotive car battery.
Each of these cells comes along with two plates which are referred to as grids. One of the grids is made of lead and the other lead dioxide.
The cells each produce energy rated at 2V, which gives a total of 12V. That is why the automotive car battery is considered a 12V battery.
The cells are independent from one another and are seen functioning quite differently although in a similar manner to produce electric current.
What Are The Difference Between Batteries And Cells?
Batteries and cells can be quite confusing, but they do not mean the same thing.
A cell can be defined as the tiniest unit of an electrochemical system. It has two electrodes.
The positive and negative, an electrolyte and one electric path that connects to the external load.
On the other hand, a battery is a collection of cells combined to increase the current output. The cells can be either connected parallel, serial or a combination of both.
Even though both are in the electrochemical class, the cell is a single electrochemical unit while the cell is a combination of the cells in either serial or parallel manner.
What Type Of Current Does A Car Battery Produce?
The car battery produces direct current after a series of reactions within the car battery itself.
The car battery is made up of chemicals such as the sulphuric acid, as well as a number of plates that lead to production of chemical reaction which then results to electric energy that is used to power the car.
The electric energy produced here is that of a direct current.
What Acid Is In Car Batteries?
Car batteries have sulphuric acid that the plates are submerged in.
This acid is designed to be the catalyst as it triggers a reaction between the two plates from each of the six plates of the car battery.
It causes the lead dioxide plate to produce ions as well as lead sulphate. The ions move on to react with the other plate and produce hydrogen and lead sulphate.
These series of reactions end up in production of electrons that move around in such a way that electricity is generated.
This is the electricity that powers the car engine, headlights, the stereo, computers and the AC system too.
The sulphuric acid is a concentration made from distilled water and which occasionally dries.
As such the acid has to be topped up regularly to prevent shortening the lifetime of the car battery.
Sources:
1. Firestone Complete Auto Care – https://blog.firestonecompleteautocare.com/batteries/how-does-car-battery-work/
2. VARTA Battery World – https://batteryworld.varta-automotive.com/en-gb/how-does-car-battery-work
3. Manbat – http://www.manbat.co.uk/news/how-do-car-batteries-work/




